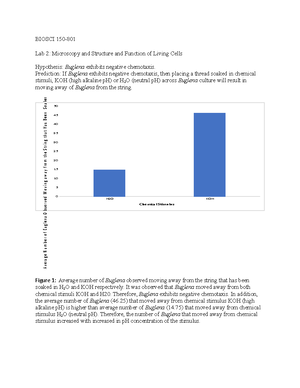
- Information
- AI Chat
Was this document helpful?
Osmosis Full Lab Report
Course: Foundations of Biological Sciences I (BIO SCI 150)
40 Documents
Students shared 40 documents in this course
University: University of Wisconsin-Milwaukee
Was this document helpful?

CING KIIM
Lab-3: Osmosis
INTRODUCTION:
Small molecules, such as water, can enter and exit the cell through spaces between membrane
molecules by diffusion. Diffusion is the process of random movement toward a state of
equilibrium. However, the cell membrane represents a barrier to substances which are either
charged or larger in size. Membranes that block or otherwise slow passage of certain substances
are said to be selectively permeable. Osmosis is the diffusion of water across the membranes.
The process depends on the relative concentration of water molecules on both sides of the
membrane.
Tonicity describes one solution’s solute concentration to that of another solution. A hypotonic
solution has a lower solute concentration than the other solution. Isotonic solutions have equal
solute concentrations. A hypertonic solution has a higher solute concentration than the other
solution. Plant cells will shrink and pulls away from the cell wall if they are placed in hypertonic
solution on the outside. The process is known as plasmolysis. In animal cells, the cells will lose
water, shrivel and have spiny appearance when they are placed in hypertonic on the outside.
Plant cells will stiffen if they are placed in hypotonic solution on the outside. They generally
retain their shape due to the presence of cell walls. However, animal cells will swell and burst if
they are placed in solution hypotonic on the outside.
Hypothesis: Water molecules will move from lower solute concentration to higher solute
concentration.
Prediction: If water molecules move from lower solute concentration, then water will move into
the dialysis tubing in bag 2 and 3 which have higher solute concentration inside the bag. Water
will move out of bag 3 since it has higher solute concentration outside the bag. The weight of
bag1 will remain the same since the bag is placed in isotonic solution.
In this experiment, the dependent variable is the change in the weight of the dialysis tubing bags.
Contents of dialysis tubing bags and contents of beakers are the independent variables.
METHODS:
For the experiment, we needed four 15-cm sections of dialysis tubing presoaked in reverse
osmosis water (RO H2O), 8 pieces of rubber bands, 4 labels, a funnel, four 400-mL beakers,
graduated cylinder to measure 10mL, paper towels, balance with weight boats, RO H2O, 15%
sucrose solution, 30% sucrose solution, and scissors.
We obtained four 15-cm long sections of dialysis tubing that had been presoaked water and
created bags by folding over one end of each dialysis tube and tied the end with a rubber band.
We also obtained four beakers, labeled the beakers, and added 200mL RO H2O to beaker 1, 2, 3
respectively. We also added 200mL 30% sucrose solution to beaker 4. With the help of a funnel
and a graduated cylinder, we filled 10mL of RO H2O,10mL of 15% sucrose solution,10 mL 30%
sucrose solution, 10mL of RO H2O to bag 1,2,3, and 4 respectively. After filling the bags with
the solutions, we smoothed out the top of the bags to remove excess air and then we folded and