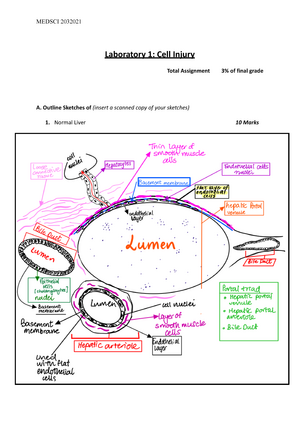
Subject: Chemistry
Which are isotopes An atom that has an atomic number of 34 and a
Subject: Chemistry
Anonymous Student
Which are isotopes? An atom that has an atomic number of 34 and a mass number of 76 is an isotope of an atom that has A) an atomic number of 32 and a mass number of 76. B) an atomic number of 34 and a mass number of 80. C) 42 neutrons and 34 protons. D) 42 protons and 34 neutrons.
Like
0All replies
Answer
The correct answer is option B. Isotopes are atoms of the same element which differ in mass number. Here the atomic number is 34 which represents the element Selenium (Se) with
Discover more from:
- Discover more from:
Related Answered Questions
- Chemistry of the Living World (CHEM 110)Relative polarity of Diflunisal moleculeAnswers
- Chemistry of the Living World (CHEM 110)Explain relative polarities between asprin, diflunisal and sodium salicylateAnswers
- Chemistry of the Living World (CHEM 110)What compounds have two DBE?Answers
- Chemistry of the Living World (CHEM 110)The equation for the combustion of proane is given by: C3H8 + 5O2 ---> 3CO2 + 4H2O The rate of the overall reaction is: Group of answer choices the same as the rate of consumption of O2. not related to the rate of consumption of O2. 5 time the rate of consumption of O2. 1/5 the rate of consumption of O2.Answers
- Chemistry of the Living World (CHEM 110)The rate constant for a given (first order) reaction is 6.4 x 10-5 s-1. Calculate the concentration of reactant remaining after 120 minutes if the initial concentration was 0.75 mol L-1.Answers
- Chemistry of the Living World (CHEM 110)Initial rate data for the reaction: 2A + B -----> C + D has been tabulated below. Find the order of the reaction with respect to B. Experiment [A] in mol L-1 [B] in mol L-1 Initial rate in mol L-1 s-1 1 0.020 0.010 1.37 x 10-3 2 0.020 0.020 5.46 x 10-3 3 0.060 0.020 1.66 x 10-2Answers