Subject: Chemistry
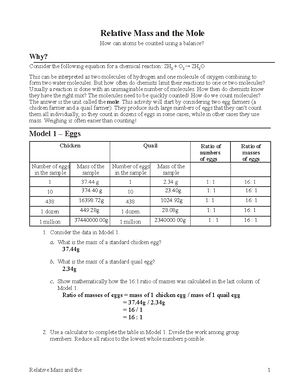
The mass of one mole of lead Pb atoms is 2072 g Use a proportion
Subject: Chemistry
Anonymous Student
The mass of one mole of lead (Pb) atoms is 207.2 g. Use a proportion to calculate the number of lead atoms in a 15.00 g sample of lead.
Like
0All replies
Answer
The mass of 1 mole of lead (Pb) = 207.2 g/ mol The mass of lead (Pb) = 15.00 g The number of moles of lead (Pb) = mass of lead / molar mass of lead
Discover more from:
- Discover more from:
Related Answered Questions
- General Chemistry I (CHM 121)
How is the mass of a single particle changed to get the mass of one mole of particles?
Answers - General Chemistry I (CHM 121)
Which sample contains more atoms, 18.016 amu of water or 18.016 g of water? Explain.
Answers - General Chemistry I (CHM 121)
If the formula mass of iron(II) sulfate (FeSO4) is 151.9 amu, what is the molar mass of iron(II) sulfate?
Answers - General Chemistry I (CHM 121)
Use a periodic table to calculate the molar mass of ammonia (NH3).
Answers - General Chemistry I (CHM 121)
How would the number of atoms in a 1.01 g sample of hydrogen compare to the numberof atoms in a 63.55 g sample of copper?
Answers - General Chemistry I (CHM 121)
How would the number of oxygen atoms in a 16.00 lbs sample compare to the number of sulfur atoms in a 32.00 lbs sample?
Answers