- Information
- AI Chat
Organic Chemistry: Dat Organic Chem Reaction Summary
ORGANIC CHEMISTRY (CHEM 008B)
Pasadena City College
Related documents
Preview text
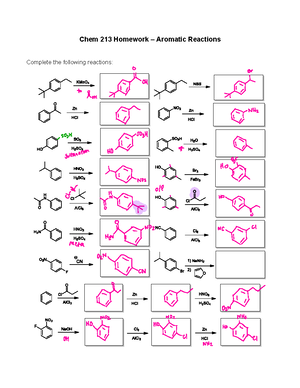
Alkene Reactions DAT Organic Chemistry Reaction Summary Sheet Hydrohalogenation Hydrohalogenation (with Rearrangement) Halogenation Hydrobromination with Peroxide Hydration Hydration (with Rearrangement) Bromination in H2O OxymercurationDemurcuration HydroborationOxidation Addition of an Alcohol Bromination in Alcohol AlkoxymercurationDemurcuration Epoxidation 1 of Catalytic Hydrogenation can also be Ozonolysis (Reducing Conditions) Ozonolysis (Oxidizing Cleavage 1. O3 2. H2O2 or O O OH 1. 2. Alkyne Reactions Catalytic Hydrogenation (Catalytic Reduction) Reduction to CisAlkene Reduction to TransAlkene Hydrohalogenation with HBr (Terminal Alkyne) Hydrohalogenation with HBr (Internal Alkyne) Halogenation with Br2 Hydration of an Internal Alkyne Hydration of a Terminal Alkyne (Markovnikov) Hydration of a Terminal Alkyne SN2 Addition of an Acetylide Ion to an Alkyl Halide SN2 Addition of an Acetylide Ion to a Ketone SN2 Addition of an Acetylide Ion to an Epoxide 2 of Diene Addition to a trans Dienophile hv or enantiomers Diene Addition to a substituted Dienophile H O H O H hv or endo (Major) O H H H O O exo (Minor) H H Grignard Reactions Addition of a Grignard Reagent to an Aldehyde O Addition of a Grignard Reagent to a Ketone O 1. H OH MgX , Ether 2. 1. MgX , Ether HO 2. Addition of a Grignard Reagent to an Ester O 1. 2 eq. O Addition of a Grignard Reagent to an Acyl Chloride O Addition of a Grignard Reagent to CO2 HO MgX, Ether HO 2. O MgX 1. CO2, Ether OH 2. Addition of a Grignard Reagent to an Epoxide (adds to the less subs. side) Addition of a Grignard Reagent to a Carboxylic Acid 2. 1. 2 eq. Cl MgX, Ether O 1. MgX , Ether Carboxylic Acid OH 2. O 1. OH MgX , Ether 2. O O MgX Carboxylate 4 of Addition of a Grignard Reagent to an Amide O NH2 O MgX , Ether 1. NH MgX 2. Deprotonated Amide Addition of a Grignard Reagent to a Nitrile 1. N O MgX , Ether Ketone 2. Electrophilic Aromatic Substitution (EAS) Reactions Alkylation (Rearrangement Possible) Cl AlCl3 Cl AlCl3 Acylation (No Rearrangement Possible) O O Cl AlCl3 Bromination Br2 Br FeBr3 Chlorination Cl2 Cl FeCl3 Nitration HNO3 NO2 H2SO4 Sulfonation SO3 H2SO4 SO3H Formylation O CO, HCl H AlCl3 5 of Acetylation of Aniline using Acetic Anhydride NH2 O H N O O pyridine Anilene O Acetanilide Hydride Reduction Reactions Reduction of an Aldehyde to a O 1. NaBH4, EtOH H 2. O 1. LiAlH4, EtOH H Reduction of a Ketone to a 2. O 1. NaBH4, EtOH OH H OH H OH 2. O 1. LiAlH4, EtOH OH 2. Reduction of a Carboxylic Acid to a O Reduction of an Ester to a O 1. LiAlH4, EtOH OH 1. LiAlH4, EtOH O Reduction of an Ester to an Aldehyde O O O OH H O H OH 2. H O Cl Reduction of an Amide to an Amine OH 2. H2O 1. LiAlH4, EtOH Cl Reduction of an Acyl Chloride to an Aldehyde 2. H 1. O Reduction of an Acyl Chloride to a 2. OH H O 1. LiAlH4, EtOH NH2 NH2 2. Hoffmann Rearrangement O 1. Br2 NH2 2. NaOH NH2 7 of Reduction of a Nitrile to an Amine N 1. LiAlH4, EtOH NH2 2. Alcohol Reactions Conversion of a to an alkyl halide via SN1 Conversion of a to an alkyl bromide via SN2 OH HX X OH HX X OH PBr3 H H OH Conversion of a to an alkyl chloride via SN2 SOCl2 H Pyridine Conversion of an Alcohol to a Tosylate Ester (OTs) OH OH Chromic Acid Oxidation of a 1o Alcohol to a Carboxylic Acid OH H Br PBr3 OH OH Dehydration of an Alcohol Br Cl H Cl SOCl2 Pyridine OTs TsCl Na2Cr2O7 or CrO3 Retention of Stereochemistry Rule O H2SO4 OH o Chromic Acid Oxidation of a 2 Alcohol to a Ketone OH Na2Cr2O7 or CrO3 O H2SO4 Chromic Acid Oxidation of an Aldehyde to a Carboxylic Acid Na2Cr2O7 or CrO3 O H PCC or DMP Oxidation of a 1o Alcohol to an Aldehyde OH H H2SO4 PCC or DMP O OH O H 8 of addition of an Alcohol to an Aldehyde or Ketone forming a O addition of an Alcohol to an Aldehyde or Ketone forming a (Protecting Group, reversed O addition of Ethylene Glycol to an Aldehyde or Ketone forming a (Protecting Group, reversed HO O C or H C or H HO C or H O O O C or H HO O OH HO C or H O O C or H Addition of a Amine to an Aldehyde or Ketone forming an Imine (Reversed O N H 2N C or H C or H Addition of a Amine to an Aldehyde or Ketone forming an Enamine (Reversed O C or H N H N C or H Double bond forms on more substituted end for Ketones Addition of a Wittig Reagent to an Aldehyde or Ketone PPh3 O C or H C or H Michael Addition to an Unsaturated Ketone O O O O or HNR2, HSR etc. Michael Addition to an Unsaturated Ketone with a Gilman Reagent (Organocuprates) O O O (CH3CH2CH2)2CuLi O 10 of Carboxylic Acid Derivative Reactions Formation of an Acyl Chloride from a Carboxylic Acid via SOCl2 O O SOCl2 Cl OH Fischer Esterification O O or Cl OH O O Alpha Reactions Self Aldol Condensation and Enone Formation O 2 H OH O O H 2O O O O NaOH H 2 Mixed Aldol Condensation and Enone Formation O H 2O H H O OH NaOH OH O H 2O NaOH O O O O O NaOH H 2O HO Self Claisen Condensation O 2 Mixed Claisen Condensation O O O O O O O 2. O O O 1. O O O O O 2. O Dieckmann Cyclization (Intramolecular Claisen Condensation) Acetoacetic Ester Synthesis O 1. O 1. O 2. Cl 3. O 4. Cl 5. O 1. O O O 2. O CO2 HO 11 of Halogenation added: 2 Br atoms Regioselectivity: Stereoselectivity: Anti Intermediate: Bromonium ion Rearrangement: Not possible Mechanism: Hydrobromination with Peroxide added: and Regioselectivity: Stereoselectivity: Intermediate: Radical Rearrangement: Not possible Mechanism: 13 of Hydration added: and OHRegioselectivity: Markovnikov Stereoselectivity: Intermediate: Carbocation Rearrangement: Possible (methyl and hydride shifts) Mechanism: Bromination in H2O added: and OHRegioselectivity: Markovnikov Stereoselectivity: Anti Intermediate: Bromonium ion Rearrangement: Not possible Mechanism: added: and OHRegioselectivity: Markovnikov Stereoselectivity: Anti Intermediate: Mercurinium ion bridge Rearrangement: Not possible Mechanism: You do not need to know the mechanism for this reaction 14 of Addition of an Alcohol added: and ORRegioselectivity: Markovnikov Stereoselectivity: Intermediate: Carbocation Rearrangement: Possible Mechanism: Bromination in Alcohol added: and ORRegioselectivity: Markovnikov Stereoselectivity: Anti Intermediate: Bromonium ion Rearrangement: Not possible Mechanism: added: and OCH3Regioselectivity: Markovnikov Stereoselectivity: Anti Intermediate: Mercurinium ion Rearrangement: Not possible Mechanism: You do not need to know the mechanism for this reaction 16 of Epoxidation added: O Regioselectivity: Stereoselectivity: Syn Intermediate: Rearrangement: Not possible Mechanism: You do not need to know the mechanism for this reaction Do know that a peroxy acid is Catalytic Hydrogenation added: 2 H atoms Regioselectivity: Stereoselectivity: Syn Intermediate: Rearrangement: Not possible Mechanism: You do not need to know the mechanism for this reaction Note: You may see Pt used as well. This is just the catalyst and does not change the outcome of the products. Ozonolysis in Reducing Conditions added: 2 O atoms Regioselectivity: Stereoselectivity: Intermediate: Rearrangement: Mechanism: You do not need to know the mechanism for this reaction Do know that the double bond gets in half, and an O atom is placed on the end of each new piece. Note: (CH3)2S is often abbreviated for dimethyl sulfide. 17 of Alkyne Reaction Details Catalytic Hydrogenation added: 4 H atoms Regioselectivity: Stereoselectivity: Anti Intermediate: Rearrangement: Not possible Mechanism: You do not need to know the mechanism for this reaction. Note: You may see Pt used as well. This is just the catalyst and does not change the outcome of the products. Reduction to added: 2 H atoms Regioselectivity: Stereoselectivity: Syn Intermediate: Rearrangement: Not possible Mechanism: You do not need to know the mechanism for this reaction Reduction to added: 2 H atoms Regioselectivity: Stereoselectivity: Intermediate: Rearrangement: Not possible Mechanism: You do not need to know the mechanism for this reaction Hydrohalogenation with HBr (Terminal Alkyne) added: 1 H atom and 1 halogen atom (can be F, Br, I, or Cl) per equivalent of HX Regioselectivity: Markovnikov Stereoselectivity: Intermediate: Carbocation Rearrangement: Possible Mechanism: The halogen goes to the C with fewer 19 of Hydrohalogenation with HBr (Internal Alkyne) added: 1 H atom and 1 halogen atom (can be Cl or Br) per equivalent of HX Regioselectivity: Markovnikov Stereoselectivity: Intermediate: Carbocation Rearrangement: Possible Mechanism: Same as for terminal alkynes, but yields a mixture of two products because both intermediates are equally stable Halogenation with Br2 added: 2 halogen atoms (can be F, Br, I, or Cl) Regioselectivity: Stereoselectivity: Anti Intermediate: Bromonium ion Rearrangement: Not possible Mechanism: Hydration of an Internal Alkyne added: 1 O atoms Regioselectivity: Stereoselectivity: Intermediate: Rearrangement: Not possible Mechanism: You do not need to know the mechanism for this reaction Do know that this reaction produces enols, which then tautomerize to form ketones. 20 of
Organic Chemistry: Dat Organic Chem Reaction Summary
Course: ORGANIC CHEMISTRY (CHEM 008B)
University: Pasadena City College

- Discover more from: