- Information
- AI Chat
Biomedical Sciences Semester 1 notes
Biomedical Sciences 2 (BIME08007)
The University of Edinburgh
Recommended for you
Preview text
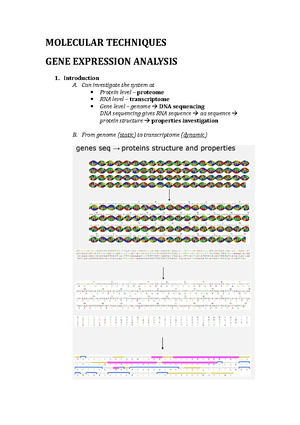
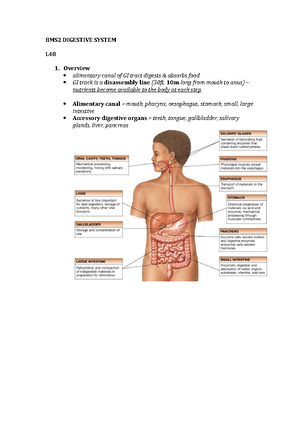
BIOMEDICAL SCIENCES 2 Semester One Lecture 2: Cell communication 1 How do cells communicate? Gap junction (simplest) – free diffusion of molecules holes in membranes between cells. Allow communication without negotiating membrane. Con = no control How do signals cross membrane? Hydrophobic (lipid soluble) Hydrophilic (water soluble) – pumps, ion channels, vesicle fusion, indirect How are signals released? Vesicle fusion – release is constitutive or evoked Ion channels – ions can be released in exchange for other ions (energy dependent). Can be constitutive or evoke Free diffusion (e. steroids and gases) can freely diffuse across membrane Paracrine – signal effects cells only in immediate environment, cell-cell communication. Can be terminated by uptake in neighbouring cells, destruction by enzymes, sequestration of extracellular matrix Autocrine – signal is released on same cell type and spread a lot further Synaptic – fast and mediated by neurons, uses mix of chemical and electrical energy. Activation at dendrite triggers electrical impulses Endocrine – hormones secreted into blood, only specific cells have right receptors Gaseous – gases can diffuse across membrane, receptor is inside Lecture 3: Cell communication 2 What are receptors? Become active when signal (ligand) binds. Signal transducers Ionotropic – allow flow of ions across membrane Metabotropic – G-protein linked. They act indirectly to regulate the activity of plasma membrane bound target Has 7 transmembrane domain structure. Ligand binding causes alpha subunit to release GDP for GTP. Through series of events activates second messengers. G-proteins are enzymes and signal is terminated by hydrolysis of GTP GENERATE SECOND MESSENGERS Kinase – similar to metabotropic, stimulate cascade. Receptors are enzymes themselves. On ligand binding site they phosphorylate themselves. Bind, activate, phosphorylation, cascade. Phosphatases terminate signal (modify existing molecules to generate new signal) Steroid receptors – used by hydrophobic molecules, can diffuse freely across plasma membrane. On ligand binding, receptors directly regulate transcription of specific genes. 2 ways of binding; both bind to DNA sequence, some live in cytoplasm and dependent on ligand binding, some always bound to DNA and need ligand binding to turn on transcription How fast are receptor responses? Ionotropic = fastest, limited by channel size and diffusion gradient Metabotropic = quite fast, limited by number of enzymatic reactions Kinase = quite fast Steroid = relatively slow, limited by speed of gene transcription, have long term effect Receptors can be targets for drugs Agonist = mimic natural ligand and activates receptor in absence of signal Antagonist = block action of ligand, inhibitory ligand function (direct or allosteric) Lecture 4: Kidney formation To what extent should we believe in a genetic blueprint, or what is a reasonable thing to mean by genetic blueprint? This is to be contrasted with self-organizations. E. birds flock together. Given that all birds are equivalent, how are they able to get so coordinated? If you are a bird you will stay an ideal distance from the bird next to you. There are only 4 or 5 simple rules but gives very complex behaviour. Blueprint driven – e. in dancing when they learn a number of steps and dances and come up with complex movements as well. Are cells in developing tissues more like dancers reading direct instructions, or like the birds following a small set of simple rules that’s not about how the final products looks, following them flexibly? Fingerprints of identical twins – monozygotic and share exactly the same genes but have very different fingerprints. The detailed anatomy of their tissues even though they have the same genes. One immediate reason to question the blueprint; if the gene specifies the anatomy as a blueprint would, the anatomy should be the same. There are lots of other examples. The problem in development is to discover how cells organise themselves to create an organ? Genetic blueprint or selforganization. Study a simple question that will explain complicated things. The example is the kidney, but the general point applies to all organs. The kidneys sit in the lumbar region of the back, they are extraordinarily rich in blood and take about 1/5th cardiac output. The artery goes into the organ in a large tree and comes out in a vein. The ureter also comes out, which takes urine out. Within the kidney there are a million units called the nephron. Functionally – the pump is the heart, push all small molecules through the filter. It takes sugar and amino acids and recovers them back into the body with ATP. Waste molecules leave the body. The nephron, the filter area is the glomerulus. There is a convoluted tubule, a long loop, another convoluted tubule (where things are recovered) and a collecting duct where the outputs of kidneys end up and the ureter takes them away. In the embryo, our kidneys form very low down between the two hind limbs. When kidneys are formed and functioning we make a third kidney called the metanephros (formed out of mesenchyme and epithelial). The epithelial tube is called the ureteric bud which forms the ureter and the collecting duct system. Surrounding them are the metanephrogenic mesenchyme that make the nephrons. Lecture 5: How Drugs Act Drug – chemical that produces a biological effect when given to a living organism. Structure must be known, not a nutrient or essential dietary requirement. FDA approved Medicine – might include nutrient or vitamin Pharmacology – the study of effects of drugs on living systems Where do drugs come from? Plants, fungi, microorganisms, synthetic chemicals. Some drugs come from animals – insulin from bozine pancreas. Biologics/biopharmaceuticals – a wide range of medicines (hormones) Specificity of drug action – must have selective action on a tissue or cell type. Interaction between drug and target must show high degree of specificity. Drug targets only recognise chemical structure of precise types. SPECIFICITY IS NEVER ABSOLUTE Selective drug action reflects specific expression patterns of protein drug targets Drugs can interact with more than one target, particularly at high doses and this gives unwanted side effects Receptor = a protein or protein complex that recognises and responds to endogenous chemical signals – e. hormone etc Measurement of body fluids. Principle: indicator – dilution principle. Method: inject known quantity of indicator (Q) and measure concentration ( C ) at selected times. Most indicators given by infusion into vein then into plasma. Takes time for metabolism so measure at different times Suitable indicators for different compartments - Intracellular fluid and interstitial fluid volume cannot be measured using a single dye but can be calculated using two. ICF = (TBW – ECF). ISF = (ECF – plasma) Why do you need two dyes? Indirect measures Indicator (dye) dilution techniques, inject quantity of indicator (Q) and measures its concentration ([C]) at selected times (t). movement of fluids, getting rid of fluids Access – suitable indicator is key, remain in compartment Plasma compartment – inject into veins Composition of compartments – made up of many different ions. Na more abundant. Why are some ions more present than others? Further reading on sodium potassium pump to make ATP. In mammalian cells, high potassium, low sodium in cells. Osmotic pressure Semipermeable membrane – has small pores that allow smaller molecules to pass but not larger Osmosis – water diffuses across the membrane from low solute concentration (high osmotic potential) to high solute concentration (low osmotic potential) Hydrostatic pressure: the pressure at a point/position in a static fluid. The higher the amount of fluid above this point, the higher the pressure will be Osmotic pressure – the pressure required to prevent osmosis. Build on this concept Osmolarity and osmolality. Molarity – moles of solute/1L of solution. Molality – moles of solute/1kg of solvent. Osmolarity – osmoles of solute/ 1L of solution. Osmolality = osmoles of solute/1kg of solvent Osmole = 1 mole. Independent of temperature Regulation of body fluid osmolality – regulation of body fluid osmolality is linked to control of total body water. It involves secretion of vasopressin (ADH) in response to an increase in osmolality or decrease in TBW (total body weight) Tonicity – describes the relative osmolality between two fluid compartments. Hypertonic (water moving out of cell, cell shrinking), isotonic (equilibrium), hypotonic (water moving into cells, cell bursting as no cell wall). Comparing two compartments Transcellular fluid about 2 litres but up to 7 litres of water secreted and reabsorbed into the gut each day Plasma colloid osmotic pressure (oncotic pressure). Oncotic pressure is an osmotic pressure inside the plasma of blood vessels. It is caused by proteins (most commonly albumin) that cannot permeate the membrane that bounds it. Filtration and reabsorptions, movement of the fluids. When hydrostatic pressure less than oncotic pressure (reabsorption), higher than oncotic pressure (filtration) General principles for ion and water balance Electroneutrality – closed compartment, the +ve = -ve. At physiological pH most proteins are negatively charged Balance between different cations and anions. Representation of inside of kidney – ions going in and out, a sequence. Gibbs-Donnan relation – if a compartment bound by a semipermeable membrane and containing impermeant ions is allowed to come to equilibrium with a surrounding compartment, the permeant ions will redistribute according to the Gibbs-Donnan relation. The compartment containing the most protein will have the highest osmotic pressure. Water will flow into the cell, potentially causing lysis. How do you calculate this? Active water transport – plays minor role at tissue level in mammals Oncotic pressure – proteins can’t cross membrane that binds to it Lecture 7: Body Fluids 2 – Cell volume regulation and fluid flows in a capillary bed Key concepts of last lecture: as a scientist we would like to measure body fluid. The indicator-dilution principle. Volume of compartment = quantity of indicator (Q)/ concentration (C). See journals on methodology. Osmolarity and osmolality. 1 osmole = 1 mole for a substance that does not dissociate in solution (for example glucose). Based on this, osmolarity = osmoles of solute. 1 L of solution (solute per water). Units: OsmL1. Osmolality = osmoles of water. 1kg of solvent (water alone). Units: Osmkg1H2O. what are the major differences between these two terms? When the temperature changes, most of the time the mass remains the same. People use osmolality as it is independent of the temperature. If the hydrostatic pressure is larger than the osmotic pressure in the capillary then filtration will happen. Gibbs-Donan relation. 5 Na and Cl ions on each side of membrane (is equilibrium) can be described as electroneutral. If there is a protein added with the Na on one side, this leads to a Na concentration gradient and the Na flows to the other side of the membrane. This causes an electrical potential across the membrane and the Cl crosses to counteract this. This is known as the Gibbs-Donnan effect. Gibbs-Donnan equilibrium equation: cation A x anion A = cation B x anion B If water flows into compartment, it may burst unless there is a sodium pump to stop this. How does the sodium pump prevent this? What is the role in the osmotic gradient in driving water across mammalian membranes? Distribution of a solute across a membrane. There are passive and active mechanisms. Active processes: carrier proteins or ion channels allow diffusion of substance and this requires energy. Passive processes: movement of particles occur through pores or even through the membrane, depending on lipid solubility. The main transport mechanisms across capillary epithelium are: filtration (which is driven by differences in osmotic pressure across the membrane) and diffusion (which is the passive movement of the subsatcnes under the influence of the concentration gradients which occurs through the pores and intracellular junctions) Routes for water movement. Some water moves through the membrane lipid but most moves through the highly selective ion channels (aquaporins). There are 13 aquaporins in humans. Aquaporin 1 (AQP1 is found in most cells). Aquaporin 2 (AQP2 is found only in the kidney). Maintenance of cell volume. Cell volume will stay constant if net water movement is prevented because there is no gradient of osmotic pressure across the cell membrane. ECF osmolality is kept remarkably constant by kidney/thirst mechanism (ADH etc.). Cell volume can be regulated by altering the cell’s solute load (osmotically active particles). Metabolism not only powers solute movement in or out of cell, but affects production/breakdown of polymers (glycogen, proteins) in cytoplasm. Primary active transport. Na/K pump. There is ECF and cytoplasm. The whole idea of the Na/K pump is ultimately 3Na ions are exported and 2K ions imported. Na binds to the Na/K pump which will stimulate the phosphorylation by ATP, causing a conformational change so that Na will leave the cell. The extracellular K will bind to the protein, triggering loss of phosphate group. This change in conformation transfers 2K into the cell. Loss of protein restores original conformation and Na sites are receptive again for the cycle to continue. In the Gibbs-Donan equilibrium we need the Na/K ATPase to regulate the cell. The Na/K ATPase pumps Na out of cell and K into cell against their concentration gradient. For every ATP molecule used there are 3 Na exported and 2K imported. Tendency of cells to swell is offset by efflux of solute across the membrane What driver water movement across cell membranes? No hydrostatic pressure gradients across mammalian plasma membranes, no significant active water transport (water moves by osmosis) Routes for water movement – some water moves through lipid (rate depends on nature of phospholipid), most moves through aquaporins Range of Aquaporins AQP1 – found in most cells, AQP2 – found only in collection ducts of kidney sensitive to ADH Maintaining cell volume – will stay constant if no gradient of osmotic pressure across membrane Receptors in brain measuring osmotic pressure and respond to it (e. by urine and thirst) Cell volume can be regulated by altering cell’s solute – metabolism powers solute movement and affects production/breakdown of polymers Role of Na pump and transporters Active transport, prevents cytoplasm coming into equilibrium with extracellular medium Generates ion pump that regulates cell volume Diffusion alone not sufficient for processes and is aided by fluid in capillary Starling’s hypothesis Lecture 8: The “Knee Jerk” Reflex One of the fastest moving areas in physiology, fundamentals of cell excitability and the nervous system. Go from a system level view back to a cellular and molecular level view of how cell excitability is controlled. Many of the things discussed are important for everything in the body. The knee jerk reflex – the basics of a simple neural circuit. The brain has several billion neurons – one of the greatest challenges in neuroscience is understanding how the brain and its connectivity works. Step back and look at simple circuit as an underlying way of how signalling works. Information flow in the central nervous system (CNS) – the brain and spinal cord. allowing information flow. The CNS receives sensory information – e. looking with eyes, listening with ears. Taking in information from outside world and from inside body. There is a sensory receptor sensing outside information – e. rods and cones in eyes detecting lighy. Sensory receptors send signals to CNS along sensory neurons which are referred to as sensory afferents. The CNS is a massive parallel computer processing information and sending out to effectors – e. skeletal muscles or glands or heart. This information that comes out comes along motor neurons or motor efferents. Unidirectional information flow. Information flow from sensory receptors to CNS to effectors. The fundamental units of the nervous system are neurons. the information flow in the whole system – have same information flow when looking at neurons. Functional unit of nervous system is the neuron. The input end in the dendrite – they receive information. the cell body or soma processes information and electrical singal is sent down axon to synapse where electrical signals are converted to chemical signalling. We have the same information flow – largely unidirectional. Real neurons: morphology related to function. We have many different types of neurons but information flow is basically the same. Efferent or motor neurons – classic cartoon neuron. Real neurons have morphology and structure related to function. Neurons form networks to transfer information. simple reflex arc; Reflex: stereotypical motor response (movement or gland secretion) to a stimulus independent of conscious thought. Knee jerk occurs at level of spinal cord – could work without head. We can build up much more complex networks but this is the simplest. Stretch of muscle activates reflex arc to cause same muscle to contract. Signal e. from the muscle at the top of leg that is sending sensory information into spinal cord, which communicates with motor neuron to send information back to muscle. The delay between sensing something and a reaction is the reaction time – 20/24miliseconds for knee jerk reflex. Doctor will ask you to cross legs – below knee cap is a tendon that is joined to muscle in top of leg. if you hit on tendon, muscle on top of leg will transiently stretch and sensory recetors are activated, talking to sensory neurons which send information to spinal cord. those sensory neruons talk directly to motor neurons which come back to the same muscle, causing it to contract. Stretch the muscle, activates motor neuron in CNS, which tells the same muscle to contract. What you should expect is for the leg to flip out. Information flow in CNS (spinal cord and brain). Components of monosynaptic reflex arc – knee jerk reflex. There is a single chemical synapse within the CNS – the connection between the sensory neuron and the motor neuron. In the CNS there is one synapse. Skeletal muscle is the effector – constantly trying to measure length and tension. It has a sensory receptor called the muscle spindle – when you hit tendon it gets stretched. This is talking to sensory neuron which is projecting into the spinal cord. at the spinal cord there is a chemical synapse talking directly to a motor neuron which comes back ot the same skeletal muscle and causes it to contract. Structure of the muscle spindle. Muscle fibre is the contracting bit – extrafusal force producing fibres. Muscle spindle sits between two tendons, intrafusal modified muscle fibre not producing force. sensory receptors that responds to stress. 10mm in humans. Muscle spindle is parallel to the force producing fibres – it is monitoring muscle length. Highly innovated – it sends information to the CNS along a sensory neuron. The alpha motor neuron comes back and innovates the force producing fibres to cause it to contract. The muscle spindle itself is controlled by the gamma motor neurons – slower. Why would you have a muscle spindle also innovated by a motor neuron? What is the problem with this system? The sensory receptor is parallel with the force producing fibres. A muscle spindle at normal length – what happens under these situations, the muscle spindle is constantly talking to the CNS by sending out action potential frequencies (tonic = very regularly spaced action potentials) to tell the length. When the muscle is stretched, the spindle increases the number and frequency of action potentials and then comes back to normal tonic level. Signalling a change in muscle length and a new muscle length. Muscles don’t usually operate when stretched -worked by contraction. If I contract a muscle – muscle spindle will become loose and floppy. Contracted a muscle – decrease the number of action potentials and spindle becomes floppy. How can you have less than 0 action potential? This is why the spindle is a modified muscle fibre. Spindle insensitive to length change when muscle contracts. Activate gamma neuron to make muscle taut again. Spindle now taut – resets new lower tonic frequency. Lower frequency of action potentials, shorter muscle. However, it can still respond. Constantly monitoring length and changing its own length – increasing its dynamic range and allowing for load compensation. Recalibrating itself to make sure it can still communicate with CNS. Muscle spindles maintain posture in response to stimulus – allows compensatory contraction to support increasing load. Muscle must contract even more to keep arm in same place when more weight is added. Transiently, the muscle is stretched which activates muscle spindle and knee jerk reflex. Same if when walking, the muscle in the knee jerk reflex constantly measuring length. Add load, system kicks in, all at spinal cord level. Components of a polysynaptic reflex arc: golgi tendon organ (GTO). What is the force and the tension of that muscle. Muscle constantly monitoring its own tension and is in series with muscle fibres. Monitors its own tension with glolgi tendon organ embedded within tendons themselves. In series with muscle fibres. This allows GTO to detect tension in that muscle. GTO communicates to sensory neuron that goes into spinal cord which will excite the next neuron down. We now talk to an inhibitory interneurone (sits within CNS and acts as a link between sensory and motor neuron). It is inhibitory because activated by sensory neruron but inhibit the motor neuron that goes back to same muscle. Converts an excitation signal to an inhibitory signal. They have different neurotransmitters controlling inhibition. As I increase tension, the sensory receptors activate the sensory neuron which will activate the inhibitory neuron which will inhibit the motor neuron. still using the membrane potential to control its physiology. When talking about membrane potential; must think about the sign, the measurement and the units. How do we set up those membrane potentials across the membrane of any cell? 3 important concepts. The ionic basis of electrical excitability; 1) unequal distribution of ions between the inside and the outside of the cell. The plasma membrane is mainly made of plasmid and membrane. The lipid membrane won’t allow anything charged to move through it; must have mechanisms such as ion channels. Essentially the plasma membrane acts as a barrier to control the ionic compostion inside and outside the cell. The extracellular composition of all our cells looks a bit like sea water. The ions inside the cell allow the biochemical processes needed for life. Potassium (K) ion. Single positive charge. Around 140mV inside the cell – more on the inside than the outside, typically 5mV. There is a big concentration gradient for K from inside to outside. A major ion that determines the resting membrane potential of most cells. Sodium (Na) has a single postivie charge. There is around 150mV outside and 15mV inside. The concentration is in the opposite direction to K. this unequal distribution of Na and K is critically important for generating action potentials. Calcium (Ca) has 2 positive charges. Important for neurotransmitter release. Its concentration is highest outside; 2mV. Has a large concentration gradient. Cells try to keep cytosol and Ca at very low levels (toxic to cells although used for signalling). 0 mM or 100nM. Chloride (Cl) has one negative charge. It has a concentration of 120mM outside and 10mM inside. The bulk solution is electroneutral (+ve charges equals -ve charges). You get a lot of negative charge from protein(-); 145mM inside and 37mM outside. Osmotic potential of intra- and extra- cellular solutions are the same. The first basic concept of how we generate membrane potentials, how we generate action potentials to do communication, is controlled by unequal distribution of ions, principally K and Na. 2) selective ion permeability. To get ions across a plasma membrane we must use ion channels for example. We have both selective and non-selective ion channels, usually for Na or K. we have selective cation channels for Na and K and selective anion channels for Cl. Important that a cell can regulate whether or not these ion channels open or close. There are also non selective ion channels which have poor discrimination between ions. However, we are focussing on selective ion channels. 3) electrochemical gradients. This is something we very often ask questions about – the idea is that there are 2 components; electrical and chemical. People think purely about the chemical component. Cell with a selective ion channels (e. K ion channel). If Na can flow through, this means the ion is permeant. In the context of K, there is a large chemical gradient from inside to outside. A large chemical gradient – ion will move outwards. Ions tend to flow from high concentration to low concentration. These ions are charge and are controlled by an electrical gradient. This moves ions towards regions of opposite charge. If there is a postiviely charged K ion, it is attracted to the negative environment. Two charges of equal sign will repel each other. The electrical gradient is dependent on the membrane potential. At rest (70mV) the inside will be negative, so a positive ion is attracted inside. However, if the inside of the cell went positive, positively charged ions are then repelled. Generally, think about the chemical gradients being constant and the thing that really changes is the electrical gradient. Electrochemical gradients drive ions through ion channels. The only thing making these ions move is the balance between the chemical gradient and electrical gradient, not using ATP. Unequal ion distribution, selective ion permeability and electrochemical gradients. A membrane impermeable to K and Cl. Has 140mM of KCl inside and 5mM KCl outside. Between the inside and outside of the cell, we have a membrane impermeable to either of these ions. All the positives and negatives cancel out, therefore there is no potential difference between those membrane = electroneutral. These membranes are completely separated, what happens if a single ion channel is opened that only allows K to go through but not Cl. K will move out to go down its chemical gradient. As soon as a single positive charge is moved out, there is now an imbalance of charge. Although it is only one charge, there is a net negative and net positive charge. What the K ion will now do is move to its electrochemical equilibrium = not its chemical equilibrium. Chemical = same concentration inside and outside. There is still a big concentration gradient, more K will move out and there will be a bigger potential difference. There is a chemical gradient from inside to out, but as some ions move out you are building at electrical gradient in the opposite direction. As the inside of the cell becomes more negative, electrical gradient for K increases and more + ions are retained inside the cell. The electrochemical gradient is combining the electrical and chemical gradients, and it is the balance that’s important. The K ion is trying to reach its equilibrium potential; there is no net flux of the ion in either direction. This is the membrane potential of the cell that would allow the chemical gradient to be balanced exactly and oppositely to the electrical gradient. The K ion is trying to move the membrane potential of the cell to a membrane potential to achieve this balance. There is a flux of ions down the chemical (Fc) and a flux down the electrical (Fe) and at the equilibrium potential these are equal and opposite. All ions are trying to reach their own electrochemical equilibrium. Can we calculate the equilibrium potential (Ex)? Simple derivation of the NERNST equation. There is a very simple relation between the equilibrium potential an ion is trying to reach and the ratio of its ionic concentrations. There are competing electrical and chemical gradients. Free energy of the chemical gradient of ion X = delta-G(chemical). Delta-G(chemical) = R T In [X]out/[X]in Where: R = gas constant 8 joules/degree T = absolute temperate in kelvin [X] = intra (in)- or extra (out)- cellular concentration of X in mM This is the calculation for the chemical gradient Free energy of electrical gradient for ion X = delta-g(electrical) Delta-G(electrical) = Z F E(x) Where: Z = valency (number and sign of charge) F = Faraday’s constant (96500 coulombs/mole) E(x) = equilibrium potential for ion x (millivolts, mV) At equilibrium: deltaG(electrical) = delta-G(chemical) ZFE(x) = R T In [X]out/[X]in Rearrange equations to get Ex (which is essentially the Nernst equation) Nernst equation; defines the equilibrium potential for any permeant ion. E(x) = RT/zF In[X]out/[X]in The concentration ratio is the important determinant. In user friendly form (at room temperate): Ex = 58/z log(10) [X]out/[X]in Using the simple Nernst equation, for K+; Ek = 58/1 log(10) [5]out/[140]in = -84mV. Its negative, -84mV, not too far away from typical resting potential of most cells, of which K is a major determinant. E(Na) = 58/1 log(10) [150]out/[15]in For K it is -84 and for Na it is +58. This is determined on whether the ions can cross the membrane. If the membrane was only permeable to Na or K it would sit at these values. The resting membrane potential of most excitable cells is largely determined by K ions. Resting membrane potential might be around -70mV and for K it is around -84mV (but never just K that is solely permeable). Equilibrium potential for Na is up at +58. If the membrane was only permeable to Na, the equilibrium potential would increase. If the membrane is only permeable to K, the equilibrium will decrease. Cell membranes behave Lecture 10: Electrical Signalling Action potentials, their fundamental features and ion channels that underly the action potential. We’ve been looking at a simple knee-jerk system and today we are looking at the ability to communicate rapidly using action potentials and electrical signalling along the sensory neuron and back down the motor neuron. Electrical communication in cells is dependent on changing the selective permeability of the membrane to different ions. Depolarization: influx of positive charge (move away from resting membrane potential). The cell might typically be at 70mV, but positive charge enters and negative environment will become more positive and depolarise (largely driven by Na). Going back towards resting potential; repolarisation = efflux of positive charge. This is due to K leaving the cell. The ability of cells to change their selective permeability (in this case to Na then to K) is fundamental to allow them to change action potentials to allow them to undergo rapid electrical signalling. This is done through ion channels, and these are voltage dependent. Any permeant ion (i. an ion that has a channel that’s selective for it) will move in a direction that will allow it to reach its own electrochemical equilibrium. When it moves it will change the membrane potential of the cell. For K, it’s equilibrium potential is -84mV. High concentration inside, low concentration outside. It will move in a direction determined by a combination of its electrical and chemical gradients. For example, if the cell was at -70mV and a K channel was opened, K would move out of the cell (losing positive charge and wants to reach -84). For Na, it would move into the cell as it wants to reach +58mV. Permeant ions move to reach electrochemical equilibrium: net flux = 0. These chemical gradients for Na or K stay relatively constant, but is dependent on the change in the electrical gradient determined by the membrane potential of the cell. Ionic basis of action potential – measuring intracellular membrane potential. This was driven by the work of Hodgkin and Huxley who used a giant squid axon (can see with the human eye). they stuck a fine electrical wire into the axon (we can’t do this in human axons as they are too small). The basic properties of action potential and the different types of voltage gated Na and K channels and their properties that allow action potential to work. Action potential: supra-threshold depolarization. Graph of membrane potential in mV and time in ms. In cardiac muscle it would be 100 times longer than this, but we are looking at neurons. An action potential is a supra-threshold depolarization. The cell at resting membrane potential is at -70mV. If there is a subthreshold or a gradient depolarization, this will allow a small depolarization. The stimulus that’s there depolarizes and then decays following the stimulus exactly. It will decay away very quickly. At this point we haven’t yet opened the voltage gated Na channels that drive the action potential. Action potentials are supre-threshold depolarizations. Give a much bigger stimulus, and make the membrane potential depolarize the threshold for action potential generation. This threshold has a particular property determined by the membrane potential at which the voltage gated Na channels start to open. If you have passed the threshold, you will see a supra-threshold response. Depolarizes very rapidly, reaches a peak, hyperpolarizes and then returns to resting state. We refer to it as an all or nothing response. if you depolarize past thr threshold you will generate an action potential. Action potentials regenerate – this ability is why you can have very fast electrical signalling from spinal cord to big toe for example. For any given neuron, an action potential typically has a very stereotyped response – every time you reach threshold you will see a very similar shape and amplitude of action potential. This depends on different types of neurons, depending on different types of ion channel that the neuron has. Stereotyped, all or nothing, suprathreshold response. Phases of the action potential and the ionic movements that occur at each phase. Depolarization (upstroke) the inside of the cell is becoming more positive. Repolarization (downstroke) inside of the cell is becoming less positive. Hyperpolarisation: inside of the cell is more negative than the rest of the cell, often very close to equilibrium potential for K. these phases are controlled by different types of ion channel. Action potential: sequential permeability changes to Na and K. the fundamental concept is that an action potential is dependent upon a sequential permeability change to Na followed by K; the increase in Na influx precedes the K that leaves (much slower at leaving than Na is at entering). We need this sequential permeability change to allow depolarization followed by repolarization. If Na and K moved simultaneously, nothing would happen to the membrane potential. This sequential change is due to different types of ion channel. 1. At rest membrane 100x more permeable to K than Na. K ion (for most excitable cells) is the major determinant for resting membrane potential. The resting potential is therefore close to the equilibrium potential for K at 70mV. 2. Depolarizing stimulus to threshold – for example at a synapse, post synaptic receptors have been activated and get small subthreshold depolarizations. If these add suffieciently, they will reach threshold (typically at 55mV) 3. Voltage gated Na channels open and Na enters down the chemical and electrical gradient. 4. Rapid depolarization toward E(Na) as Na enters the cell. Positive feedback loop = these ion channels that allow Na to flow through are activated by voltage, as you depolarize the cell more channels are opened, depolarizing more and activating more Na. this allows rapid upstroke of the action potential. The permeability of the membrane to Na now exceeds that of the permeability to K. 5. At the peak of the action potential, Na channels inactivate and Na entry declines sharply. Inactivation is not the same as Na channels closing – at +35mV the voltage to keep Na open is still there, but Na influx stops dramatically. There is part of the channel called the inactivation gate that plugs the open channel. Although the channel is open, Na can’t enter. Na permeability declines and the peak stops at this point (doesn’t get to equilibrium potential to Na). We start to open voltage activated K channels – open more slowly and need a bigger depolarization (K is starting to leave the cell) 6. K leaves the cell, causing repolarization. 7. K channels close slowly. Cell tries to reach EK, causing hyperpolarization and going below resting membrane potential. As these K channels fully close, everything is restored and we go back to resting membrane potential. Basic properties of voltage gated Na and K channels. Na channels have two gates; m gate and h gate. M gate opens is response to depolarization, causing Na to enter. The H gate blocks open Na channel, causing inactivation. The K channel has the n gate: it opens slowly with depolarization and closes slowly with repolarization. At rest, both the m gate and the n gate are both closed. With depolarization taking us above threshold, the M gate of the Na channel is opened, allowing Na to enter. The H gate then closes, blocking the channel. The N gate now opens, allowing K to leave. In hyperpolarization, the N gate is still open. The M gate converts from the inactivated state to the closed state, releasing the H gate again. Still closed, but come out of the inactivated state. At rest, the M and N gates are both closed. The system has been reset and begin again. Thinking of these properties; the hyperpolarization stage due to K leaving the cell, the properties of Na channels that allow them to inactive give us two really important properties that it confers on action potentials. The properties of the channels define the refractory period of an action period. the refractory period is the period immediately after the action potential where you cant generate another action potential or you need a bigger stimulus to generate another action potential. This is important for unidirectional action potential propagation. Thinking about the simple knee jerk reflex – if there is information going from the sensory spindle to the spinal cord you want this information to flow in one direction. If it didn’t the action potential could go backwards and forwards, no use for movement. DEPOLARIZATION: influx of positive charge REPOLARIZATION: efflux of positive charge HYPERPOLARIZATION: inside of cell more negative than rest Permeant ions move to reach equilibrium potential. Cell only permeable to K = electrochemical gradient outwards, cells only permeable to Na = electrochemical gradient inwards. Ions will move their potential. Ionic basis of action potential: measuring axons Suprathreshold depolarization - All or nothing response – high threshold and generate action potential - Can regenerate themselves, no attenuation along axon - Stereotypical response – fixed amplitude (peak typically 30mV, fixed shape duration typically 1-2ms) Changing permeability for K or Na 1. At rest membrane potential more than 100x more permeable to K than Na 2. Depolarizing stimulus to threshold 3. Voltage gated Na channels open and Na enters cell (permeability to Na changes rapidly) 4. Rapid depolarization toward ENa as Na enters cell. Positive feedback loop. Permeability of Na 10x that of K. Fast upstroke of action potential due to inflow of Na 5. Na channels inactive (h-gate), entry stops. Voltage gated K channels have slowly opened during depolarization 6. K leaves cell causing repolarization, driving forces favour K leaving cell, inside becomes more negative 7. Hyperpolarization (cells try to reach Ek). K channels close slowly 8. Cell returns to rest Properties of action potential driven by Na and K channels Voltage gated Na channel (NaV) M gate: opens rapidly with depolarization H gate: blocks open channel (inactivation) Voltage gated K channel N gate: opens slowly with depolarization, closes slowly with repolarization At rest: Sequential change in permeability critical for action potential Ion channel properties define refractory period. Allows; unidirectional action potential propagation, control of action potential frequency ABSOLUTE – period after first action potential generated another action potential. Sets absolute maximum potential neuron can generate RELATIVE – mechanism neurons use to control potential, cell hyperpolarized. Need stronger stimulus to reach threshold OUBAIN blocks Na/K pump Communication via changing action potential frequency, suprathreshold depolarization Long term maintenance of ionic gradients, Na pump uses ATP and K back in restores ionic gradient. OUBAIN blocks pump Lecture 11: Electrical Communication – conduction of action potentials The issue of unidirectional action potential propagation – sending information to CNS and then send it back. A critical determinant is the refractory period of action potentials, how they work and prevent action potentials oscillating back and forth along the same axon but allows us to go in one direction. A axon has a neuron that goes into leg muscles. The axon has to communicate within a fraction of a few ms from Edinburgh castle to the swann lecture theatre just for the sensory neuron and then go back again. The action potential direction goes from Edinburgh castle to the terminals in the swann lecture theatre. Part of the axon membrane is at rest and the K channel’s n gate is closed. The Na’s m gate is also closed. The part of the axon is that this part is -70mV relative to the outside. The depolarized or rising phase of the action potential, the Na channel is open, allowing +ve Na to enter. The axon is narrow, Na can flow in and can flow up or down. There is do discrimination in the direction it will flow. This is known as the local current flow and is involved in the regeneration of action potentials. The local current flow will start to depolarize part of the membrane and as this gets big enough it generates an action potential, allowing more Na channels to open. They can regenerate as they travel down, the local current flow depolarizes the membrane in front of it, and causes Na channels to open, cascading Na ions downwards. What about the ions flowing back? If we had Na channels that could be immediately activated, we could send depolarization in the other direction. This is where the refractory period comes in; the first thing is that part of the membrane is hyperpolarized – won’t depolarize by adding positive charge. In the absolute refractory period, the Na channels are inactivated. Even if you could depolarize this Na channel, its blocked. The refractory periods governed by the properties of Na and K channels allow unidirectional action potential transfer, which is critical for the knee jerk reflex to work. Non-invasive monitoring of neuronal function in humans. We can’t put electrodes into axons – for human axons it is very difficult to record directly from axons as they are so small compared to giant squid axons (most recordings are from cell bodies). There are ways to measure non-invasively. Clinically you cant measure action potential in a single neuron, but we can record non-invasively the activity of bundles of axons, especially in periphery nerves. Measure nerves at the base of your ankle – put an electrode at the top of the ankle and at the bottom. One electrode is stimulating a bundle of axons, and the change in action potential is measured further down. This is used to detect disorders of sensory neurons – for example Guillain-Barr syndrome, peripheral neuropathy or carpal tunnel syndrome. We are not measuring the intracellular membrane potential, we are measuring the properties of a bundle. Peripheral nerves: bundles of single neuron axons. Sciatic nerve that goes over backside. It is a nerve that comes out the spinal cord and a single axon will come out of there. In the sciatic nerve itself, there are a bundle of axons. Every single dot is the cross-section of a single axon. There are thousands of different axons altogether. We cant stick an electrode into a single axon, but we can measure from the population. This gives an action potential that we refer to as the compound action potential. Compound action potential recorded from peripheral nerve. This is fundamentally different from the action potential in yesterday’s lecture (this is the change in intracellular membrane potential of a single axon with time) whereas here this is a measurement of the activity of different axons. These are extracellular recordings; you can put the electrodes onto the skin. Within a sciatic nerve there are axons bundled together (of different sizes and functions), we can stimulate extracellularly and measure a change in membrane potential. When we stimulate upstream of measurement, we can measure the conduction velocity of action potentials in these different neuronal cell types. Plot the extracellular voltage as a function of time after stimulus. There is a complex series of changes, but each peak represents a different size and class of axon you are measuring from. The first sharp peak reflects the first action potential conduction velocities, reflecting the largest diameter of fibres. These other peaks represent the conduction velocities in other types of axon. We see conduction velocities of different speed that reflect the different types of axons in that peripheral nerve. This is looking at the speed, the amplitude tells us how many axons in each subtype. In contrast to action potentials of a single axon, these compound action potentials are graded (they are the sum of the extracellular effect of all the action potentials on all of the actions). so reach threshold and activate Na ions. Resistance to membrane is now high, so ions flow along without leaving. This is why the length constant is important. Demyelination results in slow nerve conduction, for example Multiple sclerosis or Guillan Bare syndrome. Axons are still present but Schwann cells haven’t sufficiently insulted them. The myelin sheath has been degraded, lost high resistance. Still have ion channels at nodes of Ranvier but there is a 1mm gap. As the resistance of membrane has decreased, current flow will leak out before it reaches the next node of Ranvier. Now the length constant is not optimal, it is now a fraction of a millimetre, there is not efficient coupling between nodes of Ranvier and much slower action potential conduction velocity. Very simply knee jerk reflex can be used to detect some of these changes in out sensory or motor neuron function. Today we also do measurements of conduction velocity. The change of reaction time in knee jerk reflex can be detected -slower. We have focussed on simple neural circuit, have been thinking about unidirectional information flow in CNS; how we generate electrical signals, membrane potentials, role of K, equilibrium potentials of ions, generating action potentials for rapid communication and how conduction velocity is controlled depending on fibre type and myelination. Lecture: Excitation-contraction coupling of skeletal muscle There are lots of different types of muscle in our body, namely; skeletal, cardiac (both striated) and smooth muscle. These types of muscle do not show any type of striation. Striated muscle has a striped appearance. Skeletal muscle. 40% of our body are made up by skeletal muscle (not talking about body builders). Other important features are that they are attached to the skeleton, they are responsible for locomotion. The contractility is voluntary. It requires an input from our brain to contract. Skeletal muscle are characterised by a huge number of nuclei and very large muscle cells arranged into myofibers. Cardiac muscle. Only found in the heart and the heart it is responsible for contractility. Different from skeletal muscle, it is fatigue resistance and contractions are involuntary. They are known as cardiomyocytes – they communicate with each other by forming cynsition o facilitate a pathway of excitation. Smooth muscle. Located in hollow organs such as blood vessels, respiratory tract, iris of the eye and the gastrointestinal tract. Contractions are slow and maintained and uniform. The function is to alter a level of activity of the body at a certain time. They are also fatigue resistant and activation is involuntary. Contractility of blood vessels is important to regulate blood pressure. Some of us associate going to the gym with keeping their muscles healthy. Chocolate is very good for smooth muscle health. 1. Describe the microanatomy of skeletal muscle and how this relates to function Normally the skeletal muscle is responsible for moving body parts and in order to do this is attached to skeleton directly or to the cartilage. There are exceptions such as the lips. If we were to study skeletal muscle more in depth, we would find the attachment of skeletal muscle to the tendon, which would attach muscle to the bone. Skeletal muscle is a large organ and is composed of thousands of fascicles, which are formed by thousands of single cells known as myofiber. There are other aspects that important for function; in order to contract these muscles require a high level of ATP and oxygen, they also require rich innovation because contraction is voluntary. Would expect it to be rich in blood vessels and nerves. Need a way of supporting the mesh of blood vessels and nerves around the skeletal muscle by the contribution from collagen. The collagen has a different name depending on the area; on the outside they are epimysium, surrounding the fascicles is perimysium and there is endomysium surrounding the myofibers. Their names indicate in Latin where the collagen is located. We need a membrane that separates each myofiber from its neighbour. Also has T tubules; they are important in synchrony of muscle contraction. T tubules are invaginations of cell membrane that allow the fast passage of depolarization quickly and synchronously along all the areas of the skeletal muscle myofiber. The sarcoplasmic reticulum; in order to guarantee that contraction occurs quickly and can be graded efficiently, it depends on the provision of free cytoplasmic calcium concentration, and to guarantee that calcium is always available cells have their own calcium store called the sarcoplasmic reticulum. We need energy sources, so skeletal muscles are rich in mitochondria. Myofibers are different from other cells – although their diameter is between 20-100um, they can span several cm length, making them different from even cardiomyocytes. Myofibril structure – the contractile unit that makes it up is called the sarcomere. It is a repeating functional unit. Z lines flank the end of the sarcomere. At the very centre of the sarcomere is the M line which is important as it is responsible for the structural organization of the sarcomere. Between the M line and the Z lines are the myofilaments. There are areas within the sarcomere with only one type of myofilaments, and others with two. The A band has both thin and thick filaments and are important for contraction. H zones have thick filaments and is the region where there is no overlap with thin filaments. Components of the sarcomere. What makes up the contractile side of the sarcomere. The most important feature is that they are proteins. We have myosin heads and this is part of the thick filament. The thin filament is entirely made up of actin. They are flanked by the z disk. The myofilament occupies 50% of the volume of the myofiber. The thin filaments are formed with 3 main important portions; G actin, tropomyosin and troponin complex. Why is the process of contractility not occurring all the time? It is voluntary and even when you send a signal, there are certain processes that are important to follow. Actin cannot bind the thick filament. On the head of the myosin there are binding sites for ATP; this is one of the important aspects of contractility. In this thin filament, troponin complex has a high affinity for calcium. So far… The muscle fibre are formed of contractile units known as sarcomere, and these are in turn made of proteins. These proteins are made from thin filaments and thick filaments. Proteins have important features such as binding and unbinding to ligands. These bindings produce conformational changes; the two important ligands are ATP and calcium. 2. Describe the “sliding-filament” hypothesis of contraction Between relaxation and contraction, there is a pulling of the of the myofilament to the centre of the sarcomere, it is clear that the length of the myofilament itself is not changing. Although there are contractions, there is no change in the size or the length of the myofilament. What triggers this process? 3. Describe the “excitation-contraction (EC)” coupling and how relaxation occurs Excitation contraction coupling defines the capacity of the cells to translate an action potential that is taking place in the cell membrane into mechanical work, in this case force. muscle action potential causes intracellular calcium release and contraction. Motor neurons have an end terminal, there is release of ACh and this release causes an action potential in the skeletal muscle myofiber. This is going to depolarize the cell membrane, and T tubules carry the action potential and depolarization deep into the cell interior. Importantly, T tubules carry DHP receptors that are calcium channels. When DHP receptors depolarize – as they are very close to a calcium release channel located in the internal calcium store – they are going to cause a massive release of calcium into the cytoplasm. Initiation and grading of contractility is carried out by how much calcium there is in the cytoplasm (one of the most important factors). There is calcium in cytoplasm, which produces contraction and in order for us to terminate contraction we need the calcium to return back to control level. This is achieved by a calcium pump located in the membrane of the sarcoplasmic reticulum. All the calcium that leaves should get back into the AR, terminate by calcium reuptake into SR via CaATPase. ATP is required.
Biomedical Sciences Semester 1 notes
Module: Biomedical Sciences 2 (BIME08007)
University: The University of Edinburgh
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades

This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades
Why is this page out of focus?
This is a preview
Access to all documents
Get Unlimited Downloads
Improve your grades