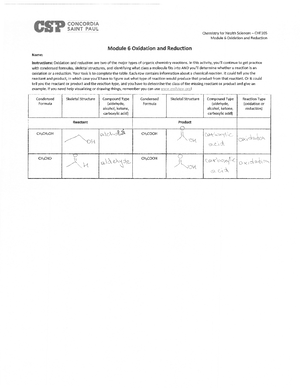
- Information
- AI Chat
Was this document helpful?
M2 Molecule-Shapes-Student-Handout for exam 1 and 2
Course: General Chemistry I (CHE 115)
27 Documents
Students shared 27 documents in this course
University: Concordia University Saint Paul
Was this document helpful?

Molecule Shapes
MODEL 1:
Molecule Shapes Simulation
(http://phet.colorado.edu/en/simulation/molecule-shapes)
PART I: ELECTRON DOMAINS
1. Explore the Model screen of the simulation. As you explore, answer the following questions.
a. How does adding an atom affect the position of existing atoms or lone pairs?
As the atoms get closer together, the bond angle keeps decreasing.
b. How does adding a lone pair affect the position of existing atoms and lone pairs?
Same as adding an atom.
2. Is the effect of adding bonded atoms and lone pairs to the central atom similar? Explain why
this could be the case.
Correct. Both types of domain take up the space. They repeal other electron domain.
3. How do the electrons in bonds (bonding domains) differ from lone pairs (non-bonding domains)?
Electrons in bonds are shared between 2 atoms. Electrons in lone pairs only belong to 1
atom.
MOLECULE SHAPES 1
We can think of a bond or a lone pair of electrons as a “domain” of electrons. Single bonds,
double bonds, and triple bonds each count as one domain.