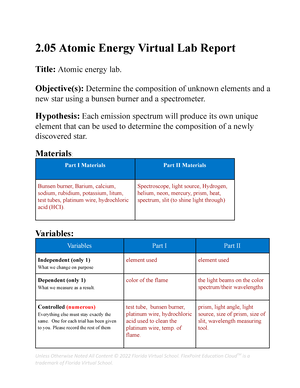
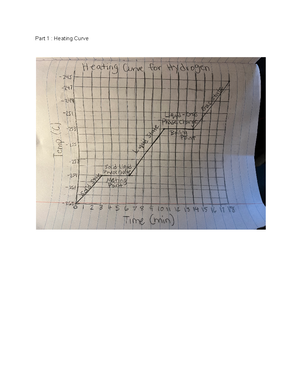
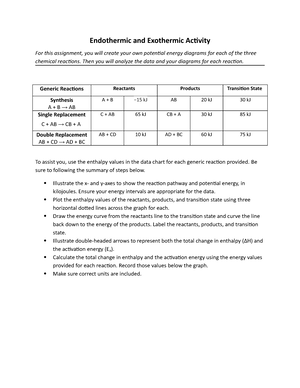
- Information
- AI Chat
Was this document helpful?

Chemistry Test 1 Notes
September
Chapter 1: Matter and Change
1.1 "Chemistry is a Physical Science"
1. What is the definition of science?
Science is the knowledge obtained by observing natural events and conditions to
discover facts and formulate laws or principles that can be verified or tested.
2. What branch of chemistry is most concerned with the study of carbon compounds?
Organic Chemistry
3. Identify each of the following as an example of either basic research, applied research, or
technological development:
a. A new type of refrigerant that is less damaging to the environment is developed.
applied research
b. A new element is synthesized in a particle accelerator.
basic research
c. A computer chip is redesigned to increase the speed of the computer.
technological development
4. What is said to be the origin of chemistry?
Alchemy
5. Should you believe any “scientific claim” you hear?
Why?
not every single one, but only the ones backed up with evidence
Students also viewed
Related documents
- 11 Light Independent Reactions njccn
- WW I - Sykes-Picot- Impact on the Middle East
- 14 Giz 9A Cell Energyyy j
- Kami Export - Student Exploration.Identifying Acids and Bases
- I actually have no idea what this one is lol hi dere i love louis tomlinson with all my heart
- Application for Employment Market Basket 3