- Information
- AI Chat
Was this document helpful?
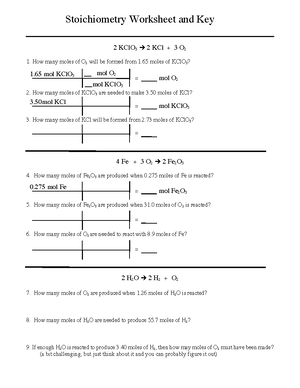
Wkst Stoich
Course: Fundamentals of Chemistry (CHM 110 )
3 Documents
Students shared 3 documents in this course
University: Triton College
Was this document helpful?

CHM 130 Stoichiometry Worksheet
The following flow chart may help you work stoichiometry problems. Remember to pay careful
attention to what you are given, and what you are trying to find.
1. Fermentation is a complex chemical process of making wine by converting glucose into ethanol and
carbon dioxide:
C6H12O6(s) 2 C2H5OH (l) + 2 CO2(g)
A. Calculate the mass of ethanol produced if 500.0 grams of glucose reacts completely.
B. Calculate the volume of carbon dioxide gas produced at STP if 100.0 grams of glucose reacts.
C. If 17.5 moles of ethanol were produced, how many moles of glucose were there in the beginning?